FAQs on Hydrogen & Methanol
What is methanol and why is it important?
Methanol (CH3OH) is a clear liquid and the simplest alcohol, with the methanol molecule containing four hydrogen atoms and one carbon atom.
While methanol is found naturally in minute quantities in things like ripe fruit, it is for the most part an important base chemical from which other chemicals and thousands of everyday products are made. About 100 million tonnes of methanol are currently produced synthetically each year mostly from natural gas or coal. Although methanol is used mainly as a chemical feedstock, methanol has a long history as a clean burning fuel and can easily be integrated into existing combustion engines.
Despite methanol today being made primarily from fossil fuels, methanol can also be made with renewable resources in which case it is referred to as green methanol.
What is green methanol?
Green methanol is methanol produced from renewable feedstocks, namely renewable power, water and carbon from the biosphere.
Green methanol is important as it can directly replace conventional, fossil based (grey) methanol. Perhaps more importantly, it can also be used as a sustainable liquid fuel for applications where alternative energy source options are limited, for example long haul shipping. In fact, the shipping industry is already preparing to convert their vessel fleets to run on green methanol as an alternative to bunker oil, LNG and other fossil fuels.
Green methanol therefore has the potential to help us transition away from a dependency on fossil fuels.
View the green methanol infographic.
What role does green methanol play in the energy transition?
Green methanol has an important role to play in the energy transition.
Green methanol can replace the chemical feedstocks traditionally acquired from fossil fuels and help to produce materials such as plastics, paint, adhesives, medicines and many other products used in modern society. Methanol can also be used to produce sustainable aviation fuel.
Green methanol can be used directly as a sustainable liquid fuel and alternative to diesel, in circumstances where electrification is not practicable.
Green methanol is already revolutionising the shipping industry, helping to achieve their net zero ambitions. Major shipping companies have ordered more than 300 methanol powered container ships with the first deliveries as soon as 2024.
How is green methanol made?
Green methanol is made by combining carbon from the biosphere with green hydrogen.
Green hydrogen is produced using renewable power and water through a process called electrolysis.
Bio-carbon is captured as CO2 either directly by removing CO2 from the air, or indirectly by the natural photosynthetic processes producing woody biomass such as roots, trunks and branches within the biosphere.
Where woody biomass is used as a feedstock, carbon contained within biomass is converted to a synthesis gas or “syngas” through a process called gasification.
Green methanol is then produced by combining green hydrogen and the syngas, where hydrogen and either carbon monoxide or carbon dioxide are reacted to produce methanol and water.
- Hydrogen and Carbon Monoxide are reacted to produce Green Methanol
- Hydrogen and Carbon Dioxide are reacted to produce Green Methanol and water.
View the infographic on how green methanol is made.
Where does the biomass come from and is that sustainable?
Our green methanol will be produced from biomass sourced exclusively from sustainable certified plantations. This biomass includes forest floor residues and processing waste from the sawmills and wood chipping plants. We will not use any biomass from old growth or native forests.
Forest floor residue consists of tree tops and small branches left after harvesting. Currently, these are often piled together and sometimes burned to enable replanting to occur, resulting in carbon being returned to the atmosphere in the process of decomposition.
Processing residues include the waste biomass from the sawmills or wood chipping plants such as saw dust, offcuts, bark and products that don’t meet customer specifications.
Our plantation biomass will include –
- Post-harvest forest floor residues which are otherwise often burned insitu.
- Woodchip processing waste such as sawdust, bark and woodchips that don’t meet export specifications.
- Saw mill waste such as offcuts, sawdust, etc.
- Bushfire damaged logs that cannot be utilised for other forestry products.
Our biomass will not include –
- Old growth forest biomass of any kind
- Native forest timber of any kind
- Primary material for which the tree was intended such as sawmill timber.
All biomass suppliers will also require internationally recognised Forestry Stewardship Council (FSC) certification or Program for the Endorsement of Forest Certification (PEFC) to ensure sustainable forestry practices are being applied.
What is biomass gasification?
Biomass gasification is a controlled process involving heat, steam and oxygen to convert plants and organic waste (known as biomass) to a gas called synthesis gas or syngas.
Because there is no combustion involved, the energy is transferred directly from the biomass to the syngas, which is made up of hydrogen, carbon monoxide and carbon dioxide.
Does green methanol also produce harmful emissions when used as a fuel?
Because the methanol molecule contains a carbon atom, when it is burned as a fuel, this carbon atom bonds with two oxygen atoms and is released as CO2. While CO2 is not directly harmful to life, for reasons explained below, it is important that only carbon extracted recently from the atmosphere (as opposed to fossilised carbon) is allowed to be released back into the atmosphere.
In the case of harmful pollutants typically generated by burning of fossil fuels, such as sulphur oxides (SOx), nitrogen oxides (NOx) and particulates, methanol contains virtually no sulphur so burning it results in negligible SOx emissions. Also, because the methanol molecule contains only one carbon atom, the particulates (soot) formed by virtue of carbon-carbon bonds when burning most fossil fuels (eg. diesel) are not formed when burning methanol.
On the other hand, burning any fuel (even pure hydrogen) in air will result in NOx emissions, because air is 78% nitrogen. However, methanol has an unusually low combustion temperature, which together with the lack of soot, makes post-combustion removal of NOx emissions much simpler than for other fuels.
Heavy fuel oil (HFO) by comparison used in the shipping industry emits black soot and is banned from use as a fuel in most many global ports due to its harmful emissions.
If green methanol emits carbon dioxide when used as a fuel, how is green methanol different from conventional fossil fuels?
The Earth’s natural carbon cycle has seen atmospheric CO2 drawn from the air over hundreds of millions of years to produce plants which then typically die and decompose, thereby returning carbon and oxygen to the air in the form of CO2 and methane. However, some plants and animals which die are buried and fossilised, preventing the return of the carbon into the atmosphere, Over eons, this has resulted in a significant reduction of the proportion of CO2 in Earth’s atmosphere, which at present is especially conducive to humans and other animals which evolved over the last few million years.
Humankind’s burning of fossil fuels over the last two hundred years has started to reverse a multi-eon process in a relatively short space of time, resulting in a rapid impact on the Earth’s climate; hence the drive to phase out further burning of fossil fuels over the next few decades.
By contrast, green methanol’s carbon lifecycle is circular. CO2 is removed from the atmosphere at the start of the cycle and released back into the biosphere at the end of the cycle. In this way, green methanol can be considered a net-zero fuel
Plantation biomass absorbs CO2 as part of the natural photosynthetic process during the 10 - 17 year growth cycle. The bulk of the harvest is used to supply the timber and paper industries, but some of the remaining biomass is used as a feedstock to produce green methanol.
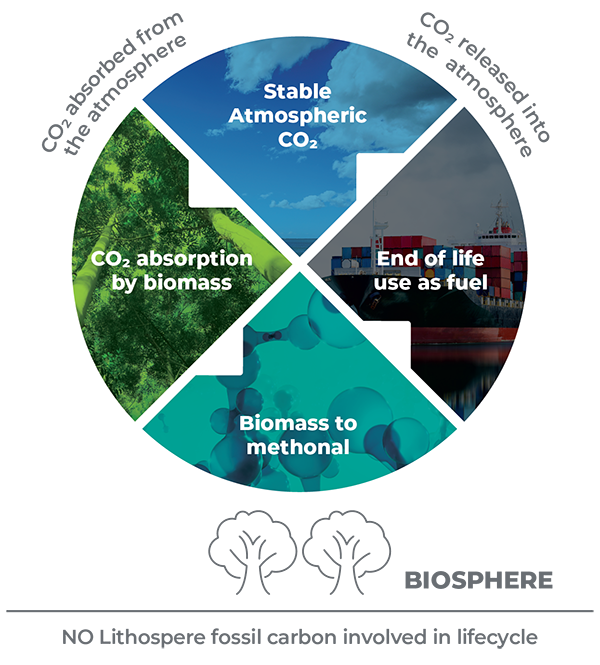
Equally significant is that when green methanol is used to make chemicals rather than burned as a fuel, it actually removes more CO2 from the atmosphere than it returns (like timber products) and therefore has a negative carbon emissions profile.
Who can use green methanol?
Green methanol has two main applications; as a sustainable chemical feedstock and as a renewable liquid fuel.
As a renewable liquid fuel, methanol can replace fossil fuels in combustion engines which presents significant opportunities for sectors where electrification is not practicable.
As a sustainable chemical feedstock, customers are likely to include existing Australian chemicals companies but may also include timber manufacturing companies where green methanol can be used to make sustainable adhesives for plywood, chipboard and laminated timber beam products.
View the infographic on who uses green methanol.
How is green hydrogen related to green methanol?
Green hydrogen is a key feedstock to produce green methanol. Green hydrogen is required to increase the ratio of hydrogen to carbon oxides before synthesis to methanol.
Green methanol is considered one of the important derivatives of green hydrogen containing more energy, being easier to handle and easier to integrate into today’s energy ecosystem.
What is green hydrogen and how is it different from normal hydrogen?
Hydrogen is the lightest element in the universe. While hydrogen is a gas in its pure form, it is nearly always found in nature as bonded to other elements. The most abundant example is water which is hydrogen is bonded to oxygen (H2O). Hydrogen also bonds easily to carbon, and hydrogen-carbon compounds are the building blocks of plants, animals, humans and all life on Earth.
Hydrogen in itself contains energy and it is possible to use hydrogen directly or in a fuel cell to power cars, trucks, turbines, etc.
While almost all of the hydrogen we use today is produced from fossil fuels, hydrogen can also be produced using renewable electricity to break the hydrogen-oxygen bonds in water, a process known as electrolysis producing green hydrogen and oxygen.
Why don’t we just use green hydrogen instead of using it to make methanol?
While hydrogen in itself contains energy and can be used in a hydrogen fuel cell or as a feedstock for chemical processes, hydrogen has some important limitations. To be used as a versatile chemical feedstock to produce medicines, materials and everyday products, hydrogen needs to be combined with carbon, and hydrogen used directly as a fuel is very challenging.
Hydrogen is a very light gas with a very low energy density. Storing hydrogen generally requires it to be liquified at very high pressures or cryogenically at a temperature close to absolute zero (-253˚C). This takes a lot of energy, and requires expensive materials and engineering.
On the other hand, by initially incurring some additional cost and energy to add carbon and oxygen to hydrogen, you end up with methanol, the simplest hydrocarbon molecule that is a liquid at room temperature, easily stored and transported with a much higher energy density with a much broader range of possible applications.
What is water electrolysis?
Water electrolysis uses electricity to split water into hydrogen and oxygen. When coupled with renewable electricity electrolysis produces what is known as green hydrogen.
There are two main mature water electrolysis technologies, Alkaline Water Electrolysis (AWE) and Proton Exchange Membrane (PEM) electrolysis.
The first AWE developments started in the early 20th century. PEM technology was developed in the 1960s. Both technologies have continued to develop since these early days to improve energy efficiency, operating life and production cost.

AWE is currently better suited to large commercial scale operation because of lower cost and a longer proven operating life. PEM electrolysis potentially offers better responsiveness to power fluctuations, which is of lower importance when grid connected. The quoted efficiencies of each technology are similar and can be optimised based on power load.
How much water is needed for green hydrogen electrolysis? Is there enough water available?
The process of electrolysis is the splitting of the water molecule into hydrogen and oxygen. However, the bulk of water used at the facility is for cooling.

